Coming Soon: Systemwide Expansion of Electronic Consents
As part of UVA Health’s ongoing efforts to streamline workflows and enhance patient care, we’re expanding the use of electronic consents (e-consents) across our system.
Currently, around 40% of perioperative cases use e-consents. Broader adoption has been limited since not all consent forms have been available electronically — until July 1. A new, systemwide initiative will complete our transition to e-consents and eliminate paper forms across key areas.
Continue reading to learn what’s changing and when — and how you can prepare.
What Is an E-Consent?
An e-consent is a digital version of a patient consent form, completed directly within Epic. Instead of filling out paper forms, providers use dropdown menus and required fields to capture all essential information. Details like the procedure name, treatment team, and date are automatically populated—reducing errors and saving time.
Required fields help ensure every e-consent meets UVA Health’s documentation standards for accuracy, completeness, and compliance.
What’s Changing?
- Vast majority of paper consents are going digital in Epic.
- New access points to consents in Epic:
- Inpatient Doctors & APPs: Look for a new “Consents” button in the Navigator activity.
- All Other Doctors & APPs: Access consents through your regular workflow or the “More” button.
- Key areas transitioning to e-consents:
- Anesthesia: Nerve Block, ICU, Dialysis, Autopsy, Surgical Procedure without Anesthesia/Sedation
- Blood Products: Combined Procedural Consent with Blood (Panel 1), Blood Transfusion, and Blood Refusal/Partial Acceptance
- Interpreter documentation: Interpreters will no longer sign consents. Instead, their name and Globo ID will be required.
- Formatting updates to improve clarity and consistency.
What’s Not Changing?
- The language and content of the consent forms remain the same.
- No changes to:
- Who is listed on the consent
- Which actions require patient consent
- Involvement of learners in the consent process
Signature Collection:
There are several options available to collect patient signatures on e-consents:
- Signature Pads: Both Bluetooth and tethered signature pads can be used.
- Click-to-Sign: Patients can sign with a single click directly on a computer using a mouse or touchpad.
- MyChart: Patients can log into their MyChart account to review and sign e-consent forms. They will receive a notification when a form is ready to sign.
- QR Code: After completing the e-consent, providers can generate a QR code from the form. Patients can scan the code with their phone or mobile device to sign electronically — no MyChart account is needed for this method.
Team members should use the signature collection options available in their department, as access may vary.
E-Consents Timeline:
The transition has already begun with an early adopter phase that launched in April. Roll out will continue with two more phases in the coming months:
Phase 1 – April 8, 2025: Complete
- Go-live for Combined Procedural and Blood e-consents in early adopter areas:
- Orthopedics
- Cardiology
- Cardiac Surgery
- Vascular Surgery
- UVA Imaging
Phase 2 –
June 17, 2025
- Users can start creating macros and exploring the workflows in advance of the July 1 go-live. The ELL tip sheet provides information on how to do this within Epic.
July 1, 2025
- Go-live for Combined Procedural and Blood e-consents across all other University Medical Center service lines
- Go-live for Anesthesia e-consents across the University Medical Center
Phase 3 – Fall 2025
- Go-live for e-consents at UVA Community Health
How to Prepare for E-Consents:
- All providers, nurses, and periop team members:
- Familiarize yourself with the changes to the Procedural Consent Panel 1-5 and Surgical Procedure e-consents.
- Familiarize yourself with new e-consents:
- Combined Procedural Consent with Blood (Panel 1)
- Blood Transfusion
- Blood Refusal/Partial Acceptance
- Anesthesia
- Nerve Block
- ICU
- Dialysis
- Autopsy
- Surgical Procedure without Anesthesia/Sedation.
- Review the ELL tip sheet on the new blood consents.
- Complete a seven-minute learning module in Workday by July 1.
This transition to e-consents marks a significant step forward in improving the accuracy, efficiency, and consistency of our consent process — ultimately enhancing the patient experience. Thank you for supporting our journey toward safer, more efficient patient care.
For answers to frequently asked questions, continue reading below.
E-Consents Frequently Asked Questions:
How long is the Procedural Consent with Blood Panel 1 good?
The consent is good for the duration of the patient’s inpatient stay or 6 months, whichever is shorter, per Informed Decision-making Medical Center Policy v.2 (see Attachment A, item #3).
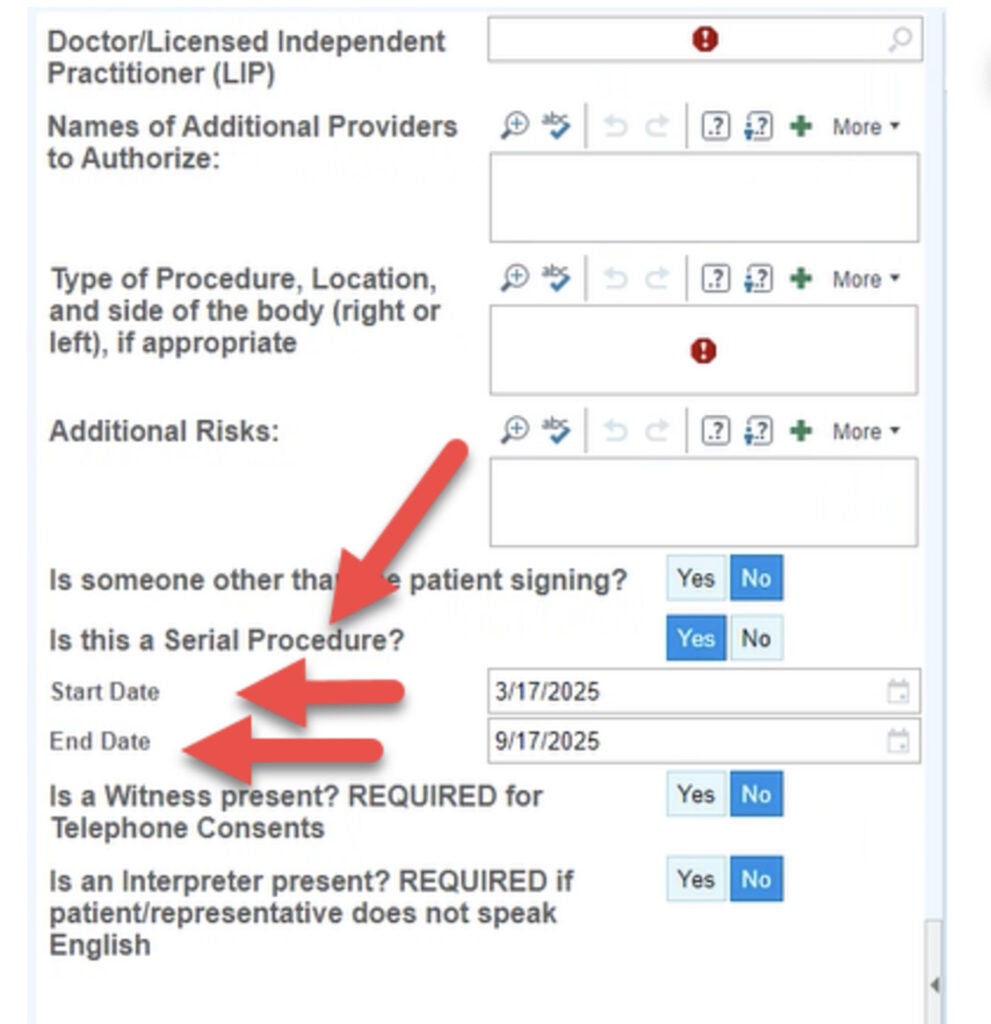
Can we do serial consents?
Serial consents may be completed using the new Procedural Consent with Blood Panel 1 e-consent form. See screenshot at right.
Will e-consents affect capacity forms for situations where in-patients lose ability/gain ability to provide informed consents?
The e-consent project will not impact the capacity form process, which will continue to be on paper. However, if a patient lacks capacity and Procedural Consent with Blood Panel 1 is needed, the legal guardian will sign the e-consent.
What are the specific changes to the Panels 1-5 and Surgical Procedure e-consents?
- In both consents, by default, the question for “Signed by other” has “No” selected.
- For pediatric patients: When a consent is loaded on a pediatric patient, the question defaults to “Yes”. This action then adds a new field and list with hard stops for the clinician to document who is signing and that person’s name.
- Interpreters will no longer be required to sign the consent. It will require the name and Globo ID of the interpreter.
- Updated UVA Health logo and standardized patient information.
- Formatting improvements for clarity.
What e-consent forms may be used for non-case-based procedures, or procedures with no case request?
All non-case-based e-consent forms are found under Miscellaneous Consents. This includes the Surgical Procedure Consent (previously listed under Procedural Consents), which should be used for cases without a case request (e.g., in-clinic procedures, bedside procedures, etc.).
What training is available to help me prepare the new e-consents?
There is a seven-minute Workday learning module and an Epic Learning Library tip sheet that cover the new blood consents.
Latest News